The global overactive bladder treatment market is expected to reach USD 4.19 Billion by 2022 from an estimated USD 3.63 Billion in 2017, at a CAGR of 2.9%. The key factors driving the growth of this market include rising prevalence of overactive bladder, aggressive marketing strategies adopted by the players in this market, and the development of innovative therapies. However, the undesired systemic effects caused by the current oral therapies may hinder the growth of this market to a certain extent.
By pharmacotherapies, the anticholinergics segment accounted for the largest share of the market in 2016
On the basis of pharmacotherapies, the overactive bladder treatment market is broadly segmented into anticholinergics, mirabegron, BOTOX, and neurostimulation. Anticholinergics being the first line of medical treatment on OAB accounted for the largest share of this market in 2016. Among the anticholinergics class of drugs, solifenacin accounted for the largest share of this market owing to its efficacy as compared to the other drugs of the same class.
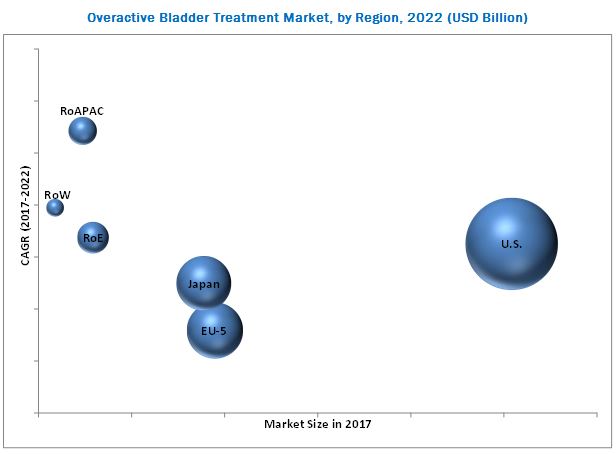
The U.S. dominated the market in 2016
The U.S. accounted for the largest share of the overactive bladder treatment market in 2016, followed by Europe and Asia-Pacific. Factors such as its well-established healthcare industry, favorable reimbursement policy, and demographics prone to bladder overactivity are contributing to the large share of the U.S. The rising prevalence of bladder overactivity, increasing geriatric population, rising healthcare expenditure, and growing awareness on the diseases due to the aggressive marketing campaigns adopted by pharma companies are majorly contributing to the growth of the European overactive bladder treatment market, which holds the second-largest market share.
The key players in the global overactive bladder treatment market are Astellas Pharma, Inc. (Japan), Pfizer, Inc. (U.S.), Allergan, Plc. (Ireland), Teva Pharmaceutical Industries Limited (Israel), Mylan N.V. (U.S.), Medtronic plc (Ireland), Endo International plc (Ireland), Sanofi (France), Hisamitsu Pharmaceutical Co., Inc. (Japan), Aurobindo Pharma Limited (India), Apotex Inc. (Canada), and Cogentix Medical, Inc. (U.S.).