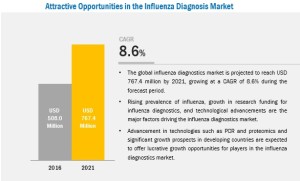
The increasing prevalence of influenza, growth in research funding for influenza diagnostics, and rising demand for faster diagnosis and control of influenza are the major factors that are expected to drive the Influenza Diagnostics Market growth in the coming years. However, variability in sensitivity and specificity among influenza diagnostic tests, presence of a complex regulatory framework for the approval of new diagnostic tests, and rising healthcare costs are expected to restrain the growth of the market to a certain extent in the coming years.
On the basis of test type, the market is segmented into traditional and molecular diagnostic tests. The traditional diagnostic tests segment is expected to account for the largest share of the global influenza diagnostics market in 2016.
On the basis of end user, the market is segmented into hospitals/clinical laboratories, reference laboratories, and other end users (point-of-care testing, home health agencies, and nursing homes).
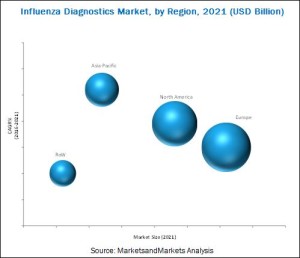
Geographic analysis reveals that Europe is expected to account for the largest share of the global market in 2016. Growth in this market can be attributed to the rapidly increasing prevalence of influenza and rise in aging population in this region. Similarly, Asia-Pacific is estimated to grow at the highest CAGR during the forecast period. The main factors driving market growth are the increasing prevalence of influenza, increased healthcare expenditure, growing demand for advanced diagnostic technologies, and government initiatives.
The major players in influenza diagnostics market include Alere Inc. (U.S.), Meridian Bioscience, Inc. (U.S.), Thermo Fisher Scientific, Inc. (U.S.), Becton, Dickinson and Company (U.S.), QIAGEN (Netherlands), Roche Diagnostics (Switzerland), and Abbott (U.S.).
Read More about Influenza Diagnostics Market:
https://www.marketsandmarkets.com/Market-Reports/influenza-diagnostic-market-222985562.html