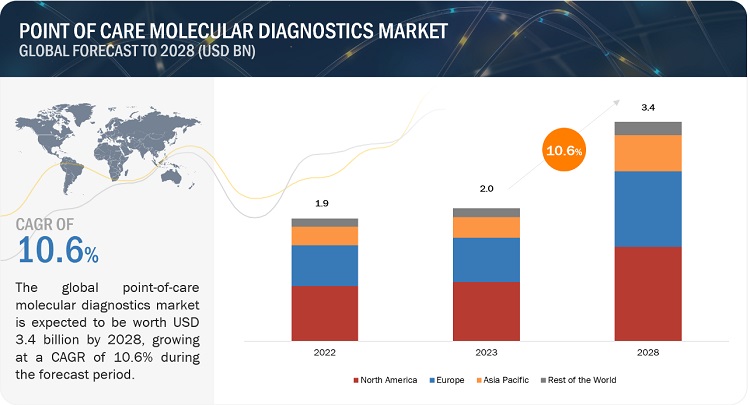
Point of Care Molecular Diagnostics Market is forecasted to grow from $2.0 billion in 2023 to $3.4 billion by 2028, with a steady CAGR of 10.6%. This report includes a thorough exploration of industry trends, detailed pricing analysis, patent reviews, and insights from key conferences and webinars. The market’s growth is propelled by the increasing incidence of infectious diseases and chronic health conditions, along with advancements in healthcare infrastructure. However, the growth is challenged by uncertain reimbursement policies and a limited number of high-complexity testing facilities.
Download an Illustrative overview: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=143524127
The Assays & Kits segment is expected to account for the largest share of the Point of Care Molecular Diagnostics Market in 2022.
The products & services in the point-of-care molecular diagnostics market are categorized into assays & kits, instruments & analyzers, and software & services. Assays & kits specifically cater to various point-of-care settings such as hospital critical care units, physicians’ offices, outpatient clinics, home care settings, and long-term care settings, among others. The continuous demand and recurring purchases of assays & kits are anticipated to fuel the growth of the point-of-care molecular diagnostics market.
The Respiratory Diseases segment accounted for the largest share of the point-of-care molecular diagnostics market in 2022.
The molecular diagnostics market is categorized into various applications, including respiratory diseases, sexually transmitted diseases, hospital-acquired infections, cancer, hepatitis, gastrointestinal disorders, and other applications. The growth of this market segment is primarily driven by the escalating prevalence of infectious diseases and the growing demand for early diagnosis and detection of these diseases. These factors are particularly significant in emerging countries, where there is a heightened need to address the challenges posed by infectious diseases through timely and accurate molecular diagnostics.
North America dominates the global point of care molecular diagnostics market.
The point-of-care molecular diagnostics market is divided into North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. In 2022, North America emerged as the leading region, capturing the largest market share in the point-of-care molecular diagnostics market. The significant share of this region can be attributed to the highly advanced healthcare systems present in the United States and Canada. In recent years, there has been a notable increase in healthcare spending across North America. According to the Centers for Medicare & Medicaid Services, national health spending is projected to grow at an average annual rate of 5.4% from 2019 to 2028, reaching a staggering USD 6.2 trillion by 2028. This growing healthcare expenditure serves as a key driver for the biotechnology sector, including the point-of-care molecular diagnostics market.
Request Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=143524127
Point of Care Molecular Diagnostics Market Dynamics:
Drivers:
- Increasing prevalence of infectious diseases and cancer
- Rising focus on decentralized diagnostics and increasing R&D funding
- Growing awareness of the early detection of infectious diseases
- Increasing use of POC diagnostic tests
Restraints:
- Unfavorable reimbursement scenario
- High capital investments and low cost-benefit ratio
Opportunities:
- Growing R&D activities in point-of-care molecular diagnostics testing
- Growth opportunities in emerging economies
Challenges:
- Stringent and time-consuming regulatory policies that significantly increase product launch cycle
- Introduction of alternative technologies
Key Market Players:
The major players operating in this market are Abbott Laboratories (US), F. Hoffmann-La Roche Ltd. (Switzerland), bioMérieux SA (France), Danaher Corporation (US), Quidel Corporation (US), QIAGEN N.V. (Netherlands), Co-Diagnostics, Inc. (US), Biocartis NV (Belgium), Meridian Bioscience, Inc. (US), Thermo Fisher Scientific, Inc. (US), Lucira Health, Inc. (US), Cue Health (US), OpGen, Inc. (US), Binx Health, Inc. (US), Molbio Diagnostics Pct. Ltd. (India), Genomadix (Canada), Visby Medical, Inc. (US), QuikPath PTE Ltd. (Singapore), MD-Bio (US), QuantuMDx Group Ltd. (UK), Aidian Oy (Finland), GeneSTAT Molecular Diagnostics, LLC (US), Labsystems Diagnostics Oy (Finland), Akonni Biosystems (US) and Curetis N.V. (Germany).
Get 10% Free Customization on this Report: https://www.marketsandmarkets.com/requestCustomizationNew.asp?id=143524127
Recent Developments:
- In April 2023, QIAGEN N.V. (Netherlands) launched QIAstat-Dx in Japan with a respiratory panel for syndromic testing.
- In June 2022, Biocartis NV (Belgium) launched the Rapid CE-marked IVD Idylla GeneFusion Panel for fast treatment decisions in lung cancer.
- In May 2022, bioMérieux SA (France) received De Novo FDA Authorization for its BIOFIRE Joint Infection (JI) Panel.
- In September 2021, F. Hoffmann-La Roche Ltd. (Switzerland) acquired TIB Molbiol (Germany) to expand its PCR test portfolio with a wide range of assays for infectious diseases.
Point of Care Molecular Diagnostics Market Advantages:
- Rapid Results: One of the key advantages of point of care molecular diagnostics is the ability to deliver rapid results. Traditional laboratory-based molecular testing often involves lengthy turnaround times due to sample transportation and processing. Point of care molecular diagnostics, on the other hand, provides real-time results, enabling healthcare providers to make timely decisions regarding patient care and treatment.
- Portability and Accessibility: Point of care molecular diagnostic devices are typically compact, portable, and easy to use. This portability allows for testing to be performed at the patient’s bedside, in clinics, or even in remote or resource-limited settings. It improves accessibility to diagnostic testing, particularly in areas where laboratory infrastructure is limited or nonexistent, leading to faster diagnosis and treatment initiation.
- Improved Patient Management: Point of care molecular diagnostics facilitates more efficient and streamlined patient management. Rapid and accurate diagnosis at the point of care enables healthcare providers to initiate appropriate treatment plans promptly. It reduces the need for multiple follow-up visits and unnecessary treatments, resulting in better patient outcomes and reduced healthcare costs.
- Infectious Disease Control: The rapid identification and control of infectious diseases are critical in preventing their spread. Point of care molecular diagnostics enables timely detection of pathogens, such as bacteria or viruses, allowing healthcare providers to initiate appropriate infection control measures promptly. This is particularly important in settings such as hospitals, long-term care facilities, and outbreak situations.
- Personalized Medicine: Point of care molecular diagnostics plays a vital role in advancing personalized medicine. It allows for the detection of specific genetic markers or mutations associated with diseases, enabling tailored treatment decisions based on an individual’s unique genetic profile. This enhances treatment efficacy and reduces the risk of adverse reactions to medications.
- Cost-Efficiency: While the initial investment in point of care molecular diagnostic devices may be higher, they can lead to long-term cost savings. By providing rapid results at the point of care, unnecessary hospitalizations, repeat visits, and unnecessary treatments can be avoided. This not only reduces healthcare costs but also improves resource allocation and efficiency within the healthcare system.
- Research and Surveillance: Point of care molecular diagnostics also contributes to research and surveillance efforts. These devices facilitate the collection of real-time data on disease prevalence, drug resistance patterns, and emerging infectious agents. This data can aid in monitoring disease outbreaks, guiding public health interventions, and informing research initiatives.
Overall, the point of care molecular diagnostics market offers advantages such as rapid results, portability, improved patient management, infectious disease control, personalized medicine, cost-efficiency, and its contribution to research and surveillance. These benefits have the potential to transform healthcare delivery, particularly in settings where immediate diagnostic information is critical for effective decision-making and patient care.
Content Source:
https://www.marketsandmarkets.com/PressReleases/point-of-care-molecular-diagnostic.asp


