Viral inactivation is an important and mandatory step in the manufacturing process of biological products to remove or inactivate potential contaminant viruses.
Viral inactivation testing is necessary by regulatory authorities for investigational new drug (IND) submission and is mainly critical in process development for biologicals including tissue and tissue products, stem cell products, cellular and gene therapy products, blood and blood products, and vaccine and therapeutics.
Download PDF Brochure (Viral Inactivation Market):
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=121427017
Research Methodology Adopted for Viral Inactivation Market:
A combination of bottom-up and top-down approaches was used to calculate market sizes and growth rates of the global market and its subsegments.
Secondary information was used to identify overall revenue, geographic reach, and product portfolios of Viral Inactivation Market players. Estimates of their viral inactivation market segment revenues were validated through primary interviews. Primary interviews with key opinion leaders were also used to determine the percentage shares of each subsegment and the relative differences in growth rates
Global Viral Inactivation Market Based On;
· Method
· Product
· Application
· End-user
· Geography and more
Speak to Our Subject Expert (Viral Inactivation Market):
https://www.marketsandmarkets.com/speaktoanalystNew.asp?id=121427017
Global Viral Inactivation Market Major Segmentation in Detail;
The product segments included in the report are kits and reagents, services, and viral inactivation systems and accessories.
Based on end-user this Viral Inactivation Market is categorized into pharmaceutical and biotechnology companies, contract research organizations, academic research institutes, and other end users. Other end user segment primarily includes cell banks, small cell culture laboratories and consultants, microbiology laboratories, immunology laboratories, molecular laboratories, animal facilities, toxicology laboratories, and media/sera manufacturers.
The method segments included in the report are the solvent detergent method, pasteurization, and other methods. Other viral inactivation method includes low pH, microwave heating, irradiation, and high-energy light.
Request Research Sample Pages (Viral Inactivation Market):
https://www.marketsandmarkets.com/requestsampleNew.asp?id=121427017
Major Players in The Viral Inactivation Market:
Clean Cells (France), Charles River Laboratories International, Inc. (U.S.), Danaher Corporation (U.S.), Merck KGaA (Germany), Parker Hannifin (U.S.), Rad Source Technologies (U.S.), Sartorius AG (Germany), SGS S.A. (Switzerland), Texcell, Inc. (France), Viral Inactivated Plasma Systems SA (Switzerland), and WuXi PharmaTech (Cayman) Inc. (China).
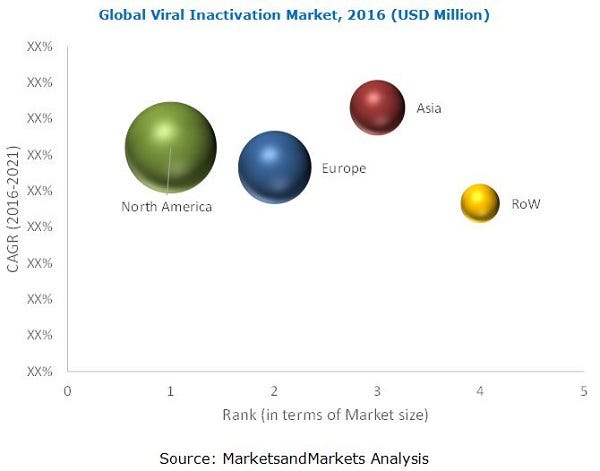
The Geographic segments included in this report are Asia, Europe, North America, and the Rest of the World (RoW). The North America segment is further divided into Canada and the U.S. Asia segment is further divided into China, Japan, India, and Rest of Asia.


