According to the new market research “Real World Evidence Solutions Market by Component (Dataset (Claims, Clinical, Pharmacy, Patient), Services), Therapeutic Area (Oncology, Cardiovascular, Immunology), End User (Pharmaceuticals, Medical Devices, Payers, Providers) – Global Forecast to 2023″, analyzes and studies the major market drivers, restraints/challenges, and opportunities. The global real-world evidence market is valued at 612.0 million in 2017 and projected to reach USD 1,348.1 million by 2023, at a CAGR of 14.3%.
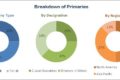
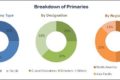
The global Real World Evidence Solutions market is segmented based on component, therapeutic area, end user and regions
On the basis of component, the data sets segment accounted for the largest share of the global real world evidence market. The large share can be attributed to factors such as easy availability of massive amounts of data, increasing dependence of outcome-based studies on real-world data, and rising demand for information by payers and providers regarding drug safety.
Download a PDF Brochure:- https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=76173991
Browse and in-depth TOC on in “Real World Evidence Solutions Market ”
92 – Tables
40 – Figures
157 – Pages
Based on therapeutic area, the oncology segment accounted for the largest share of the real-world evidence market in 2017. The large share of this segment can be attributed to the high number of clinical trials conducted for oncology and the rising prevalence of cancer worldwide.
Based on end user, the pharmaceutical & medical device companies segment accounted for the largest share of the real world evidence market. The large share of this segment can be attributed to the increasing importance of RWE studies in drug approvals, the need to prevent costly drug recalls, and the increasing need to assess drug performance in real-world settings.
Request Free Sample Report @
https://www.marketsandmarkets.com/requestsampleNew.asp?id=76173991
North America accounted for the largest share of the real world evidence market in 2017, followed by Europe and Asia Pacific. Presence of a favorable regulatory environment, high number of RWE service providers, the presence of a well-established pharmaceutical industry in the region, coupled with the high R&D expenditure, are the major factors responsible for the large share of North America in the global real world evidence market.
Market Players
The key players in the Real World Evidence Solutions market are IQVIA (US), ICON (Ireland), PAREXEL (US), Pharmaceutical Product Development (US), Optum (US), International Business Machines Corporation (US), Cognizant (US), Oracle (US), SAS (US), Syneos Health (US), Anthem (US), Clinigen Group (UK), Palantir Technologies (UK), and Flatiron Health (US).
View Complete Press Release @
https://www.marketsandmarkets.com/PressReleases/real-world-evidence-solution.asp