The rising focus on end-to-end RWE services and growth opportunities in emerging markets are expected to provide further growth opportunities for players operating in the real world evidence market. However, the reluctance to rely on real-world studies and the lack of universally accepted methodological standards for data collection some of the factors limiting the growth of this market during the forecast period.
The global real-world evidence market is projected to reach USD 1,348.1 million by 2023 from USD 689.9 million in 2018, at a CAGR of 14.3% during the forecast period.
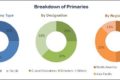
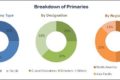
On the basis of component, the data sets segment accounted for the largest share of the global market in 2017. The large share can be attributed to factors such as easy availability of massive amounts of data, increasing dependence of outcome-based studies on real-world data, and rising demand for information by payers and providers regarding drug safety.
Download a PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=76173991
Based on therapeutic area, the real-world evidence market is broadly categorized into oncology, neurology, immunology, cardiovascular disease, and other therapeutic areas. The oncology segment accounted for the largest share of the market in 2017. The large share of this segment can be attributed to the high number of clinical trials conducted for oncology and the rising prevalence of cancer worldwide.
Based on end user, the real world evidence market is segmented into pharmaceutical & medical device companies, healthcare providers, healthcare payers, and other end users (academic institutions, patient advocacy groups, and health technology assessment agencies). The pharmaceutical & medical device companies segment accounted for the largest share of the market in 2017.
North America dominated the real world evidence solutions market in 2017
The real-world evidence market is broadly segmented into four major regions, namely, North America, Europe, Asia Pacific, and the Rest of the World (RoW). In 2017, North America accounted for the largest share of the market, followed by Europe and Asia Pacific. Presence of a favorable regulatory environment, high number of RWE service providers, the presence of a well-established pharmaceutical industry in the region, coupled with the high R&D expenditure, are the major factors responsible for the large share of North America in the global real world evidence market.
Request a Sample Pages @ https://www.marketsandmarkets.com/requestsampleNew.asp?id=76173991
Leading Companies in Real World Evidence Market
The prominent players in the global real world evidence market are IQVIA (US), International Business Machines Corporation (US), ICON (Ireland), PAREXEL (US), Pharmaceutical Product Development (US), Optum (US), Cognizant (US), Oracle (US), SAS (US), Syneos Health (US), Anthem (US), Clinigen Group (UK), Palantir Technologies (UK), and Flatiron Health (US).
To speak to our analyst for a discussion on the above findings, click Speak to Analyst