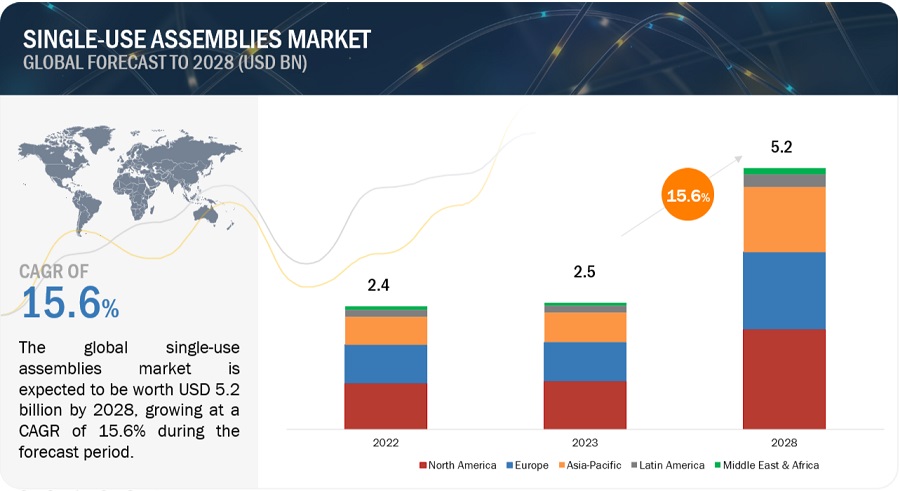
Single Use Assemblies Market in terms of revenue was estimated to be worth $2.5 billion in 2023 and is poised to reach $5.2 billion by 2028, growing at a CAGR of 15.6% from 2023 to 2028 according to a new report by MarketsandMarkets™. The global single-use assemblies market is growing owing to factors such as rising adoption of single-use assemblies among startups and SMEs, development and launch of technologically advanced single-use products that offer streamlined workflows, portability, and rapid implementation, and increasing demand for single-use assemblies for R&D and biologics manufacturing. The market growth could be hampered by regulatory concerns and significant concerns regarding extractables and leachables arising from the components of single-use assemblies in bioproduction.
Download an Illustrative overview:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=46226549
Browse in-depth TOC on “Single Use Assemblies Industry”
235 – Tables
44 – Figures
272 – Pages
The bag assemblies segment accounted for the largest share by product in the single-use assemblies industry in 2022.
By product, the single-use assemblies market has been further categorized as bag assemblies, filtration assemblies, bottle assemblies, mixing system assemblies, and other products. The bag assemblies segment held the largest share of the global single-use assemblies market in 2022. The increasing use of bioprocessing applications is driving the growth of the single-use bag assemblies segment. These bags enable critical liquid handling in biomanufacturing processes and eliminate the time & cost of additional cleaning-in-place and sterilization-in-place (CIP/SIP) system setup, maintenance, and validation.
The standard solutions segment accounted for the largest share of the solution segment in the single-use assemblies industry in 2022.
Based on solutions, the global single-use assemblies market has been segmented into standard solutions and customized solutions. The standard solutions segment held the largest market share in 2022. The pharmaceutical and biopharmaceutical industries have adopted standard solutions extensively, driven by the benefits they offer. These advantages encompass enhanced manufacturing process efficiency, lowered capital costs, increased flexibility through the utilization of pre-qualified assembly components, reduced implementation time, and greater adaptability in production planning.
The filtration & purification segment accounted for the largest share of the application segment in the single-use assemblies industry in 2022.
The single-use assemblies market is segmented into filtration & purification, aseptic transfer, cell culture & mixing, formulation & fill-finish, storage, and sampling based on application. Filtration & purification is the largest application segment of the single-use assemblies market. Factors such as cost efficiency, flexibility and scalability, reduced risk of cross-contamination, and technological advancements in single use assemblies used for filtration & purification are expected to drive the filtration & purification segment during the forecast period.
The North American region catered for the largest share of the single-use assemblies industry in 2022.
The single-use assemblies market is segmented into North America, Europe, the Asia Pacific (APAC), Latin America (LATAM), and the Middle East and Africa (MEA). North America regional market held a substantial share of the single-use assemblies market owing to the strong presence of the pharmaceutical industry, increased research and development (R&D) spending, the expansion of biosimilars markets, the rising number of drug development projects, and stringent regulations for the pharmaceutical industry.
Request Sample Pages:
https://www.marketsandmarkets.com/requestsampleNew.asp?id=46226549
Single Use Assemblies Market Dynamics:
Drivers:
- Increasing adoption of single-use assemblies among startups and SMEs
- Rapid implementation and low risk of cross-contamination
- Growing biologics and biosimilars market
- Cost savings with single-use assemblies
- Technological advancements
Restraints:
- Regulatory concerns
- Issues related to leachables and extractables
- Leakage and integrity issues
Opportunities:
- Emerging markets
- Rising focus on increasing bioprocessing capacities among biopharmaceutical companies
Challenge:
- Standardization of single-use assemblies
- Disposal of waste
- Demand and supply gap
Key Market Players of Antibody Drug Conjugates Industry:
Key players in the single-use assemblies market include Thermo Fisher Scientific Inc. (US), Sartorius AG (Germany), Danaher (US), Merck KGaA (Germany), PARKER HANNIFIN CORP (US), Saint-Gobain (France), Repligen Corporation (US), Corning Incorporated (US), Entegris (US), Meissner Filtration Products, Inc. (US), NewAge Industries (US), Antylia Scientific (US), Lonza (Switzerland), Romynox (Netherlands), SaniSure (US), Keofitt A/S (Denmark), Intellitech, Inc. (US), Dover Corporation (US), Foxx Life Sciences (US), TSE Industries, Inc. (US), Fujimori Kogyo Co., Ltd. (Japan), Michelin (France), Cellexus (Scotland), and Fluid Flow Products, Inc. (US).
Recent Developments:
In February 2022, Sartorius Stedim Biotech (France) acquired the chromatography division of Novasep (France). The portfolio acquired comprises chromatography systems primarily suited for smaller biomolecules.
In August 2022, Thermo Fisher Scientific opened a new single-use technology site in Tennessee, which has 400,000 square feet of floor space. It became the company’s largest SUT site in its growing network.
Single Use Assemblies Market Advantages:
- Flexibility and Scalability: Single-use assemblies are highly adaptable, allowing for rapid changes in production processes and easy scalability from small-scale research and development to large-scale manufacturing. This flexibility reduces downtime and enables efficient production adjustments.
- Cost-Efficiency: Traditional stainless steel equipment requires significant capital investment, maintenance, and cleaning validation. Single-use assemblies eliminate these costs by avoiding the need for cleaning, sterilization, and long-term maintenance, making them a cost-effective choice, especially for smaller batch production.
- Reduced Cross-Contamination Risk: Disposable components minimize the risk of cross-contamination between batches, ensuring product purity and quality. This is crucial in industries like biopharmaceuticals, where maintaining product integrity is paramount.
- Time Savings: Single-use assemblies streamline production processes by eliminating time-consuming cleaning and sterilization steps. This results in faster batch turnover and shorter production lead times.
- Improved Product Safety: Single-use assemblies reduce the risk of contamination by eliminating the need for manual cleaning and sterilization processes, ensuring a higher level of product safety and consistency.
- Environmental Benefits: Some single-use assemblies are designed with sustainability in mind, featuring recyclable or biodegradable materials. This aligns with growing environmental concerns and corporate sustainability goals.
- Compliance and Validation: Single-use assemblies simplify regulatory compliance and validation processes because they offer consistent and traceable components. This simplifies documentation and ensures compliance with industry standards.
- Lower Capital Investment: Industries can avoid large capital investments in stainless steel equipment and facilities by adopting single-use technology, making it more accessible for startups and smaller companies.
- Space Savings: Single-use assemblies require less physical space compared to traditional stainless steel equipment, allowing for more efficient use of manufacturing facilities.
- Innovation and Customization: Manufacturers continually innovate and customize single-use assemblies to meet specific process requirements, offering a wide range of options tailored to different applications.
Overall, the single-use assemblies market’s advantages make it an attractive option for industries seeking efficient, cost-effective, and flexible solutions while meeting rigorous quality and safety standards.
Related Links: