Aseptic fill finish manufacturing is a vital component in pharmaceutical production, particularly for sterile drug products such as injectables, biologics, and vaccines. This process involves filling and packaging drugs in a contamination-free environment, ensuring that the final product is sterile and safe for patient use.
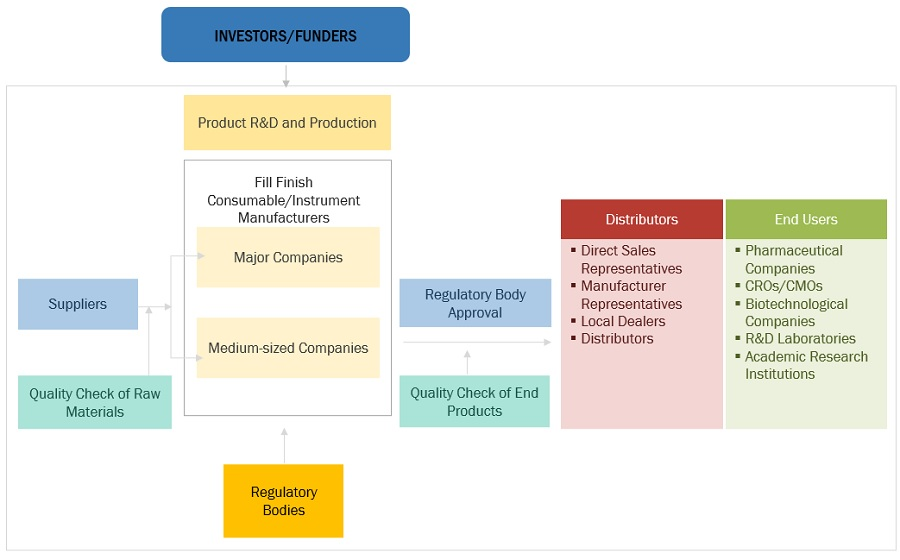
Key Components of Aseptic Fill Finish Manufacturing:
- Sterilization: The process begins with sterilizing all equipment, drug components, and packaging materials. Sterilization ensures that no contaminants are introduced during the fill finish process. Methods like steam sterilization, gamma irradiation, and filtration are commonly used.
- Filling and Sealing: Drugs are filled into sterile containers such as vials, syringes, or ampoules in an aseptic environment. Sealing the containers immediately after filling is crucial to maintain sterility. Advanced isolators and Restricted Access Barrier Systems (RABS) are often used to create these sterile zones.
- Quality Control: Every batch undergoes rigorous quality testing to ensure compliance with regulatory standards, including tests for particulate matter, sterility, and endotoxin levels. This guarantees that the aseptic fill finish manufacturing process meets stringent FDA and EMA guidelines.
- Automation and Robotics: Recent advancements in automation and robotics have minimized human intervention, reducing the risk of contamination. Automated systems improve precision and efficiency in both filling and sealing operations, making them more reliable.
- Regulatory Compliance: Aseptic fill finish manufacturing is heavily regulated. Pharmaceutical companies must adhere to Good Manufacturing Practices (GMP) and comply with FDA, EMA, and other regulatory bodies’ guidelines. This ensures that the drugs produced are both safe and effective for patients.
Importance of Aseptic Fill Finish Manufacturing:
In the pharmaceutical industry, aseptic fill finish manufacturing is a critical process for ensuring product sterility, especially for biologics and other sensitive products. It protects patients from potential infections and complications by maintaining the integrity of the sterile drug products. Moreover, aseptic processing is crucial in meeting the growing demand for biologics and injectable drugs in the global healthcare market.
Conclusion:
Aseptic fill finish manufacturing is an indispensable process in the pharmaceutical industry, ensuring that sterile drug products are produced safely and effectively. By maintaining a contamination-free environment through advanced sterilization techniques, automation, and stringent regulatory compliance, this process plays a critical role in delivering high-quality injectables, biologics, and vaccines to patients. As demand for sterile pharmaceuticals continues to grow, advancements in aseptic technologies will be essential to meet global healthcare needs, ensuring patient safety and product integrity. For pharmaceutical companies, mastering the aseptic fill finish process is not just about quality—it’s about ensuring trust in their products.
By understanding and prioritizing the complexities of aseptic fill finish manufacturing, pharmaceutical manufacturers can confidently meet regulatory standards while improving operational efficiency and patient outcomes.
Content Source:
https://www.marketsandmarkets.com/Market-Reports/fill-finish-manufacturing-market-6249609.html
https://www.marketsandmarkets.com/PressReleases/fill-finish-manufacturing.asp

