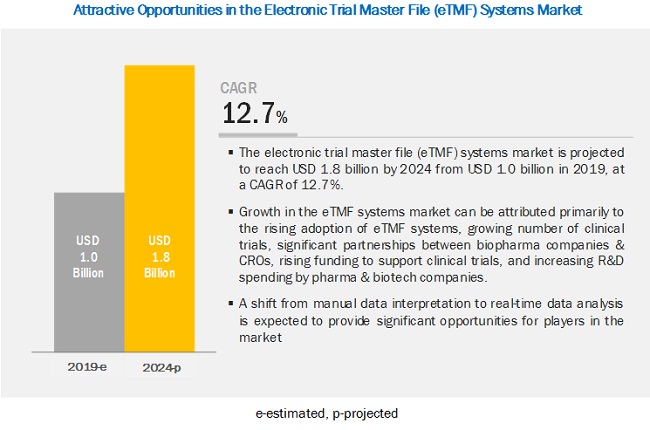
Factors such as rising adoption of Electronic Trial Master File (eTMF) Systems, rising number of clinical trials, partnerships between biopharma companies and CROs, rising funding to support clinical trials, and the growth in the R&D spending by pharma & biotech companies are driving the growth of the eTMF Systems Market.
The Electronic Trial Master File Systems Market is projected to reach USD 1.8 billion by 2024 from USD 1.0 billion in 2019, at a CAGR of 12.7%
The availability of substantial R&D budgets with large pharmaceutical & biotechnology companies will drive the adoption of the eTMF systems among pharmaceutical & biotechnology companies.
Based on end-user, the eTMF systems market is segmented into pharmaceutical & biotechnology companies, contract research organizations (CROs), and other end-users (medical device companies, academic research institutes, and consulting service companies). The pharmaceutical & biotechnology companies segment accounted for the largest market share in 2018. The increasing applications of eTMF software in clinical project management and the availability of substantial R&D budgets with large pharmaceutical & biotechnology companies will drive the adoption of eTMF systems in this end-user segment
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=94357456
The heavy dependence of end-users on service providers will drive the services segment in the eTMF systems market
Based on the component, the Electronic Trial Master File Systems Market is segmented into services and software. The services segment accounted for the largest market share in 2018. The large share of this segment can be attributed to their indispensable nature and repetitive requirement. End-users of eTMF systems rely heavily on service providers for consulting, data storage, implementing services, training, maintenance, and regular upgrades of solutions.
On the basis of Delivery Mode, the electronic trial master file (eTMF) systems market has been segmented as follows:
# Cloud-based eTMF
# On-premise eTMF
Geographically, North America accounted for the largest share of the market in 2018. The large market share of North America in the market can be attributed to the increasing government funding for clinical research, a large number of clinical trials, and the presence of several major players in the US.
Get 10% Customization on this research report:
https://www.marketsandmarkets.com/requestCustomizationNew.asp?id=94357456
Veeva Systems (US), Oracle Corporation (US), Phlexglobal Limited (UK), TransPerfect Global Inc. (US), Aurea Software (US), LabCorp (US), ePharmaSolutions (US), Wingspan Technology, Inc. (US), MasterControl (US), SureClinical, Inc. (US), Dell EMC (US), Paragon Solutions (US), PharmaVigilant (US), Mayo Clinic (US), Database Integrations, Inc. (US), CareLex (US), Ennov (France), Forte Research (US), Freyr (US), Montrium (US), NCGS Inc. (US), SAFE-BioPharma (US), SterlingBio Inc. (US), BIOVIA Corp. (US), and arivis AG (Germany) are the key players in the Electronic Trial Master File (eTMF) Systems Market

