The In Vitro Diagnostics (IVD) reagents market is experiencing significant growth, driven by technological advancements and an increasing emphasis on early disease detection. As of 2023, the market was valued at approximately USD 57.29 million and is projected to reach USD 87.41 million by 2029, exhibiting a compound annual growth rate (CAGR) of 7.7%.
Key Market Drivers
- Technological Innovations in Molecular Diagnostics: Advancements in molecular diagnostics have enhanced the accuracy and efficiency of diagnostic tests, leading to increased adoption of IVD reagents. These innovations facilitate early detection and personalized treatment strategies, improving patient outcomes.
- Rising Awareness of Preventive Healthcare: There is a growing emphasis on preventive healthcare, with individuals and healthcare providers focusing on early disease detection to improve prognosis and reduce treatment costs. This shift has led to increased utilization of IVD reagents in routine screenings and diagnostic procedures.
- Increasing Prevalence of Chronic Diseases: The global rise in chronic conditions such as diabetes, cardiovascular diseases, and cancer has escalated the demand for diagnostic testing, thereby propelling the IVD reagents market.
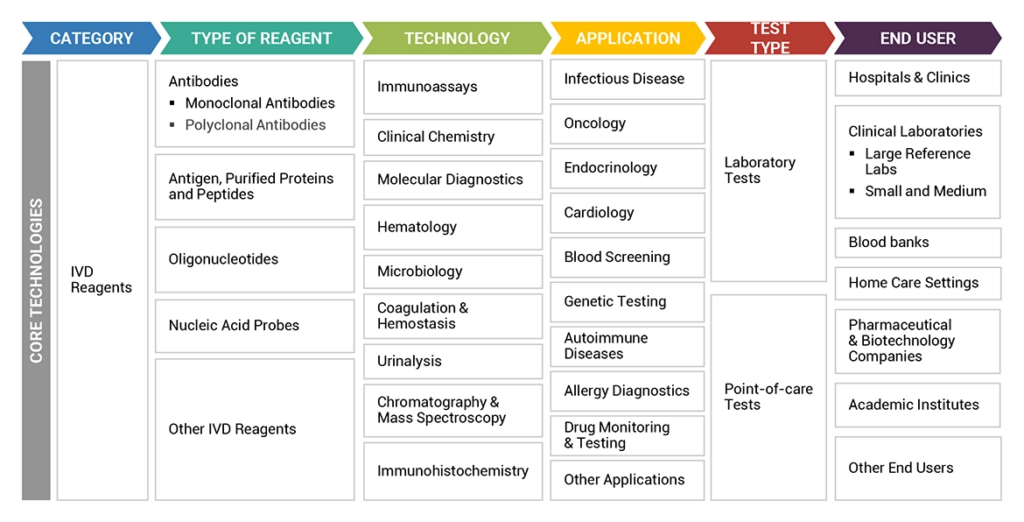
Regional Market Insights
- North America: Dominating the market, North America is projected to reach USD 32.7 billion by 2029, growing at a CAGR of 6.8%. Factors contributing to this growth include a well-established healthcare infrastructure, high adoption of advanced diagnostic technologies, and increasing healthcare expenditure.
- Asia Pacific: Anticipated to exhibit the highest growth rate with a CAGR of 10.0% during the forecast period, the Asia Pacific region’s expansion is attributed to a rapidly increasing geriatric population, rising prevalence of chronic diseases, and growing investments in healthcare infrastructure.
Emerging Trends and Opportunities
- Shift Toward Point-of-Care Testing and Automated Analyzers: There is a notable shift toward point-of-care (POC) testing, which offers rapid results and enhances patient compliance. The expansion of tests available through POC testing reagents has significantly increased, supporting informed clinical decisions and improving healthcare outcomes.
- Rising Significance of Companion Diagnostics: Companion diagnostics are becoming increasingly important as they assist healthcare providers in making treatment decisions based on the best response to therapy. The co-development of companion diagnostics with therapeutic products can significantly alter the drug development process, yielding safer drugs with enhanced therapeutic efficacy.
- Growth in Proteomics and Genomics Research: The expansion of proteomics and genomics research has led to the development of novel diagnostic tests, further driving the demand for IVD reagents. These fields offer insights into disease mechanisms, enabling the creation of targeted diagnostics and personalized medicine approaches.
Challenges
- Stringent Regulatory Requirements: The IVD industry faces stringent regulatory requirements, with evolving standards across regions. In the U.S., the FDA regulates IVD products under 21 CFR 809, requiring manufacturers to submit 510(k) applications for device modifications. Similarly, the European Union has transitioned to the In Vitro Diagnostic Regulation (IVDR), necessitating CE marking for market entry. These regulatory frameworks can pose challenges to market entry and product launches.
- High Costs of Advanced Diagnostic Tests: The substantial costs associated with advanced diagnostic tests can limit accessibility, particularly in low- and middle-income regions. This economic barrier may hinder the widespread adoption of innovative IVD reagents.
Competitive Landscape
The IVD reagents market is highly competitive, with key players focusing on product innovation, strategic partnerships, and mergers and acquisitions to strengthen their market position. Companies are investing in research and development to introduce advanced diagnostic solutions that cater to the evolving needs of healthcare providers and patients.
Conclusion
The IVD reagents market is poised for substantial growth, driven by technological advancements, increasing awareness of preventive healthcare, and the rising prevalence of chronic diseases. Stakeholders in the healthcare industry should focus on leveraging emerging trends such as point-of-care testing and companion diagnostics to capitalize on growth opportunities. However, navigating regulatory challenges and addressing cost barriers will be crucial for sustained market expansion.
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=131261429

