The research report provides insights into the global molecular cytogenetics market. It provides valuable information on the products, applications, and regions in the market. The geographic analysis for these segments is also presented in this report. Leading players in the market are profiled to study their device offerings and understand strategies undertaken by them to be competitive in this market.
The above-mentioned information would help the buyer understand market dynamics. In addition, the forecasts provided in the report will enable firms to understand the trends in this market and better position themselves to capitalize on the growth opportunities.
High Cost of Advanced Instruments Will Restrain the Growth of this Market;
Molecular Cytogenetics Market
The implementation of molecular cytogenetics in clinical & research laboratories requires high capital investments. The instruments needed for cytogenetics research and procedures are expensive since they are equipped with advanced features and functionalities. Owing to their high costs, companies and research institutes with smaller R&D budgets cannot afford to purchase or produce such expensive instruments.
On the other hand, pharmaceutical companies will require many such systems to maintain efficiency, and hence their capital cost increases significantly. Furthermore, academic research laboratories find it difficult to invest in such systems as they have controlled budgets. In developing countries owing to budget constraints, small- and medium-sized hospitals and clinical pathology laboratories cannot afford high priced, fully automated, technologically advanced instruments.
Request Research Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=148469224
Expected Revenue Surge: The molecular cytogenetics market is projected to reach USD 2.52 Billion by 2021 from USD 1.55 Billion in 2016, at a CAGR of 10.1%
Geographical Scenario in Depth:
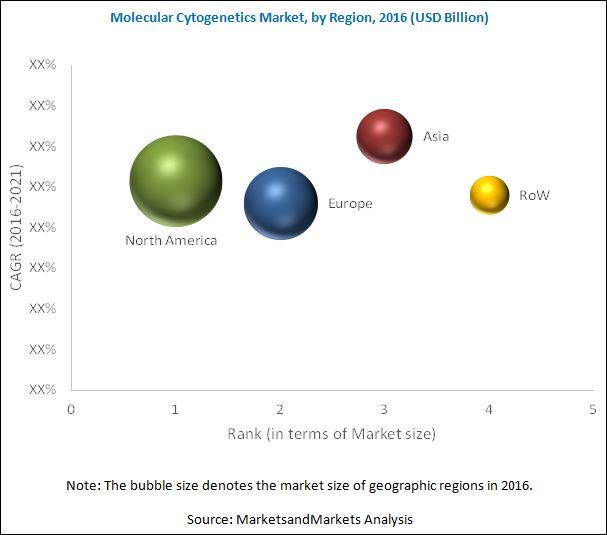
The global molecular cytogenetics market is segmented into North America, Europe, Asia, and the Rest of the World (RoW). North America is expected to account for the largest share of the market during the forecast period. Growth in this regional segment is driven by factors such as increase in the aging population and increasing prevalence of cancer and genetic disorders in the region.
Leading Key-Players:
The Key players in the molecular cytogenetics market include F. Hoffmann-La Roche Ltd. (Switzerland) Danaher Corporation (U.S.), Thermo Fisher Scientific, Inc. (U.S.), Abbott Laboratories (U.S.), Agilent Technologies (U.S.), PerkinElmer, Inc. (U.S.), Illumina, Inc. (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Oxford Gene Technology (U.K.), and Applied Spectral Imaging (U.S.).
F. Hoffmann-La Roche Ltd. (Switzerland) was the largest player in the global molecular cytogenetics market, with a share of 15.2% in 2015. The company has a strong presence in the U.S. and in regions such as Europe, the Middle East, and Africa (EMEA), Asia-Pacific, Latin America, and Japan. It focuses on acquisition as its key growth strategy. For instance, in January 2014, Roche acquired Genia Technologies, Inc. (U.S.). This acquisition strengthened Roche’s next-generation sequencing pipeline. Moreover, in February 2015, Roche acquired Signature Diagnostics AG (Germany). This acquisition allowed Roche to further develop non-invasive treatment and monitoring devices for cancer patients.
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=148469224
Major Molecular Cytogenetics Market Developments;
– In January 2014, Roche acquired Genia Technologies, Inc. (U.S.). This acquisition strengthened Roche’s Next Generation Sequencing pipeline.
– In February 2015, Roche acquired Signature Diagnostics AG (Germany).
– In January 2016, Thermo Fisher Scientific, Inc. acquired Affymetrix (U.S.). This acquisition strengthened Thermo Fisher Scientific’s leadership into bioscience business and create new market opportunities in genetic analysis.
– In July 2016, Thermo Fisher Scientific and HEALTH BioMed (China) collaborated to support HBM’s development of molecular diagnostic kits for infectious diseases and pharmacogenomics screening which will serve the Chinese market.


