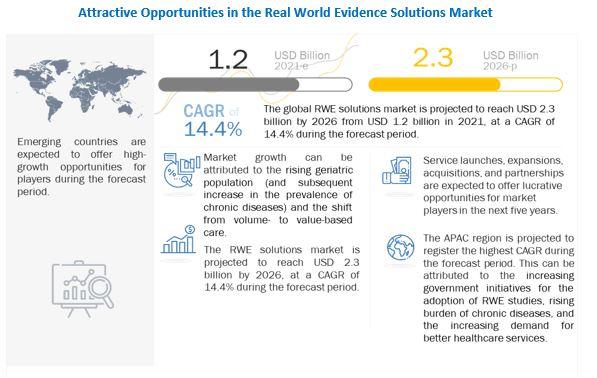
The rising geriatric population (and the subsequent increase in the prevalence of chronic diseases) is a key factor driving the growth of this market. The shift from volume- to value-based care, delays in drug development (and the subsequent increase in development costs), growth in R&D spending, and support from regulatory bodies for the use of RWE solutions are some of the other major factors that are driving the growth of this market.
[215 Pages Report] Real-world data and real-world evidence are used to monitor the post-market safety and adverse events of drugs. The monitoring of this data assists in making regulatory decisions. The healthcare community is using RWE and RWD to support coverage decisions and to develop guidelines and decision support tools for use in clinical practice.
The emergence of this pandemic has posed severe financial constraints on pharma-biopharma companies in several countries. In this regard, RWE solutions have proven to be very helpful, as they allow industrial and academic researchers to monitor patients using digitally connected platforms while helping to organize and evaluate clinical data for regulatory submissions.
Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=76173991
RWE is set to become the most influential emerging technology to help in the fight against the COVID-19 outbreak, according to the latest poll on GlobalData’s Pharmaceutical Technology website. In this poll, which was completed by 935 of its readers in April 2021, more than one-third of the respondents indicated that RWE would have the greatest impact on the management of COVID-19. A smaller percentage of the readers identified telemedicine (28%) and artificial intelligence (20%) as the most important emerging trends.
With almost 80% of clinical trials failing to meet their initial enrollment projections, delays in patient recruitment can cripple a trial and R&D program. Healthcare providers are increasingly investing in health information technologies, which is permitting them to better track and connect with patients. Hence, patient trial enrollment can come to the point of care.
Several companies are prioritizing the use of RWE to improve hypothesis development/trial design and accelerate trial recruitment. For instance, in January 2020, IQVIA (US) collaborated with AP-HP. This collaboration helped IQVIA to increase complex clinical trials and real-world evidence studies within France and Europe.
COVID-19 Impact on the Real World Evidence Solutions Market
The emergence of this pandemic has posed severe financial constraints on pharma-biopharma companies in several countries. In this regard, RWE solutions have proven to be very helpful, as they allow industrial and academic researchers to monitor patients using digitally connected platforms while helping to organize and evaluate clinical data for regulatory submissions.